Metals vs. Non-metals vs. Metalloids: The periodic table contains all of the elements discovered on the earth. These elements are classified into different categories on the basis of their physical and chemical properties. Metalloids, Metals and non-metals are those elements. If we talk about metalloid Vs. metal Vs. Non-metal differences, both are different from each other in appearance, malleability, conductivity, electronegativity etc.
Let’s take a closer look at Non-metals vs. Metals vs. Metalloids
| Electronegativity | Metals have the lowest electronegativity than non-metal and metalloid |
| Metallic Behavior | Only metals and metalloids possess metallic behavior |
| Conductors | Only Metals and metalloids are good conductors |
| Look | Metals have a shiny look; non-metals are dull, while metalloids can be dull and shiny. |
Table of Contents
What are Metals?

If we talk about metals meaning: these are the type of elements which are shiny, hard, malleable and readily form positive ions. All metals are good conductors of electricity and denser than the other elements. One of the best properties of metals is that they can be recycled without even changing their properties. Indium, Bismuth, Lead, Aluminium, Gallium, and Nihonium are some basic metals.
What Are Non-metals?

If we talk about Non-metals meaning: these are the elements with dull appearance and present on the right side of the periodic table. All of these elements have low boiling and melting points. They are also not a good conductor of electricity and gain electrons in chemical reactions. Carbon, oxygen, selenium, sulfur, hydrogen, neon, helium, Krypton, Xenon, and Argon, are all non-metals. Some non-metals like helium and hydrogen make up almost 99 per cent mass of the universe.
What Is Metalloid?

If we talk about metalloids meaning: these are the elements which possess the properties of metals and non-metals. Which means they have both dull and shiny appearances and exist in various forms. They are sometimes good conductors of electricity and sometimes not, which makes them known as “semiconductors”. Metalloids play a big role as semiconductors as they are used in some essential electronic devices like computers. They are also used to make batteries, ceramic and polymers. Silicon, Arsenic, Germanium, and Boron are some common metalloids.
5 Key Differences Between Metals, Non-metals and Metalloids
| Components | Metals | Non-Metals | Metalloids |
|---|---|---|---|
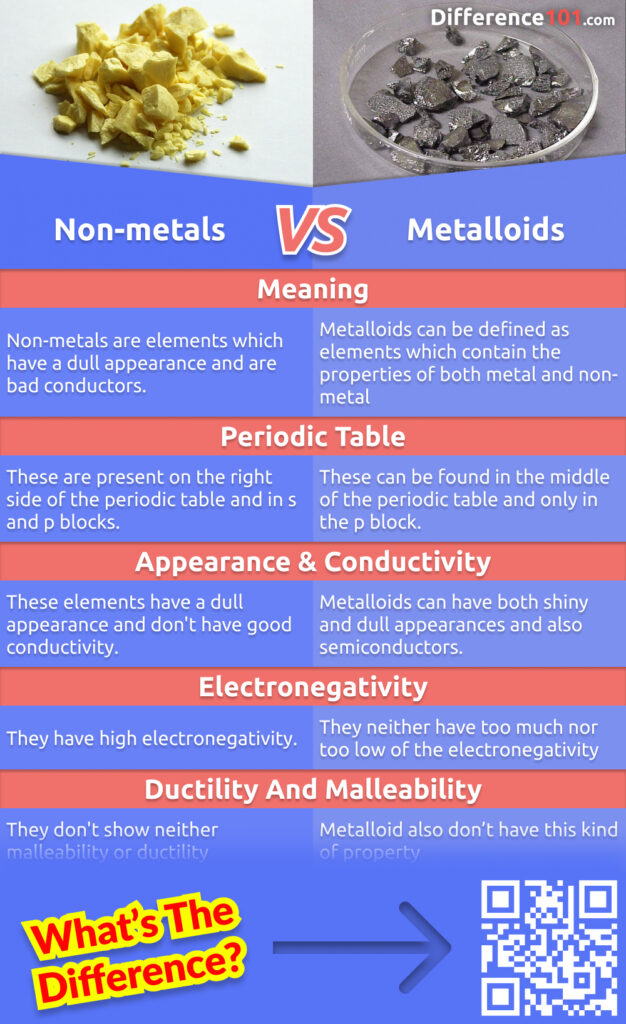
| Meaning | Metal can be defined as an element with a shiny appearance and good conductivity. | Non-metals are elements which have a dull appearance and are bad conductors. | Metalloids can be defined as elements which contain the properties of both metal and non-metal |
| Periodic Table | These can be found on the left side of the period table and s, p, d, f, blocks. | These are present on the right side of the periodic table and in s and p blocks. | These can be found in the middle of the periodic table and only in the p block. |
| Appearance & Conductivity | Metal is well known due to its shiny appearance and good conductivity. | These elements have a dull appearance and don’t have good conductivity. | Metalloids can have both shiny and dull appearances and also semiconductors. |
| Electronegativity | They have a very low electronegativity | They have high electronegativity. | They neither have too much nor too low of the electronegativity |
| Ductility and malleability | Metals are the elements which show malleability and ductility | They don’t show neither malleability or ductility | Metalloid also don’t have this kind of property |
Metalloids vs. Metals vs. Non-metals vs. Similarities
- Whether it is metal, non-metal or metalloid, all of these are elements present on the periodic table.
- All three elements are classified on the basis of their physical and chemical properties.
Metalloids vs. Metals Vs. Non-Metals Examples
Metals Examples
- Calcium
- Iron
- Zinc
- Nickel
- Sodium
Non-Metals Examples
- Iodine
- Helium
- Argon
- Sulfur
- Bromine
Metalloids Examples
- Boron
- Tellurium
- Arsenic
- Tennessine
Metals vs. Non-Metals vs. Metalloids Pros and Cons
Metals Pros and Cons

Pros of Metals
- Metal alloys are used for manufacturing purposes and for building structures.
- Alloys containing metal, whether just one, are stronger and more reliable than single metals.
Cons of Metals
- Manufacturing metal parts means relying on heavy metal suppliers, which makes it very expensive. Which makes it difficult for businesses to buy these.
- Sometimes it becomes very difficult to create complex pieces from metals, as comes the viscosity of the metal.
Non-Metals Pros and Cons

Pros of Non-Metal
- Hydrogen, a non-metal, Is an important ingredient in vegetable ghee and ammonia production. This ammonia is further used as a fertilizer for plants.
- Carbon is an essential non-metal for the growth and development of the living organism as it is a basic component of proteins, enzymes, carbohydrates and vitamins.
Cons of Non-Metals
- Some of the non-metals have a tendency to harm plants by reducing the pH of the soil.
- Non-metals like nitrogen oxide and sulfur dioxide can generate smog by combining it with water and smoke. This smog can be very dangerous as it can cause a risk of asthma and lung infection.
Metalloids Pros and Cons

Pros of Metalloids
- Metalloids play an important role in diverse physiological processes in plants. And some metalloids like selenium, silicon and boron are essential for the proper growth of plants.
- Metalloids are very famous in modern industry as well. Some metalloids like silicon and germanium are used as semiconductors.
Cons of Metalloids
- Some of the metalloids have toxic effects on the organism. Which can cause serious threats to animals, plants and microorganism.
- Some toxic metalloids and metals can cause neurological damage, fetal abnormalities, immune system suppression, and cancer.
Metals vs. Non-Metals Comparison Chart

Non-Metals vs. Metalloids Comparison Chart
Comparison Video
Conclusion
Metals, non-metals and metalloids all are elements present on the periodic table. All three elements are classified on the basis of their physical and chemical properties. The main difference between metals and non-metals and metalloid is that metals are hard and have a shiny appearance. Non-metals have opposite properties of metals, and metalloids have the properties of both metal and non-metals. For example, metals are good conductors, non-metals are bad conductors, and metalloids are semiconductors. And if we compare metals versus non-metals versus metalloids electronegativity, metals have very low electronegativity, non-metals have very high, while metalloids neither have very low nor too high electronegativity. All of these three elements are an important part of our daily life and even essential for proper living.







