The biggest trigonal planar vs. trigonal pyramidal difference lies in the presence of a lone pair of electrons in the central atom of the latter and its absence in the former. Additionally, trigonal planar displays bond-bond repulsion while trigonal pyramidal displays both bond-bond and bond-lone pair repulsion.
Let’s take a closer look at Trigonal planar vs. Trigonal pyramidal:
| Lone pair | Pyramidal has a lone pair of electrons as against planar |
| Plane | Atoms of the planar lie in one plane while that of pyramidal doesn’t |
| Bond angle | Pyramidal generally makes a bond angle of 107° as against 120° made by planar |
| Geometry | Planar is an sp2d geometry while pyramidal is an sp3d geometry |
Table of Contents
What is Trigonal Planar?
Trigonal planar is a molecular geometry model in chemistry that has one atom at the center and three peripheral atoms forming an equilateral triangle. All the atoms of a trigonal or triangular planar species lie in the same plane and all bond angles formed are of 120°.
What is Trigonal Pyramidal?
Trigonal pyramidal is a molecular geometry model in chemistry that has one atom at the apex and three atoms at the base forming a tetrahedron. The atoms of a trigonal pyramidal species do not lie in the same plane and all bond angles formed are less than 109° (the angle of a tetrahedron). Generally, this bond angle is about 107°.
Trigonal Pyramidal vs. Trigonal Planar Similarities Explained
- Both these terms are used to describe three-dimensional geometries in chemistry.
- Both trigonal planar and pyramidal are made up of molecules having four atoms.
- Both the structures are made up of a central atom with three atoms in the corners forming a triangle.
6 Key Differences Between Trigonal Planar and Trigonal Pyramidal That You Must Know
There are some key differences between trigonal planar and trigonal pyramidal that are listed here:
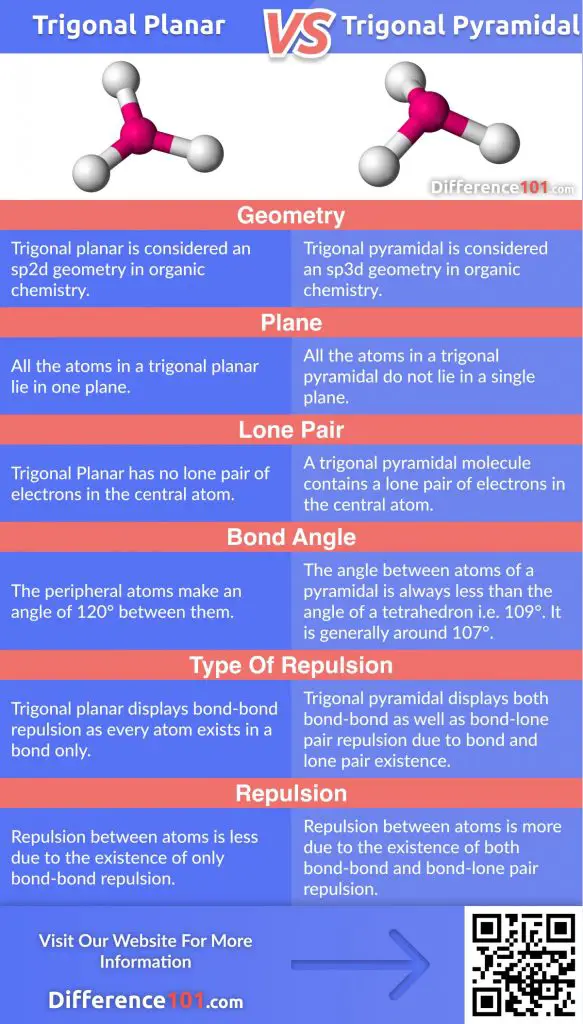
| Basis | Trigonal Planar | Trigonal Pyramidal |
|---|---|---|
| Geometry | Trigonal planar is considered an sp2d geometry in organic chemistry. | Trigonal pyramidal is considered an sp3d geometry in organic chemistry. |
| Plane | All the atoms in a trigonal planar lie in one plane. | All the atoms in a trigonal pyramidal do not lie in a single plane. |
| Lone Pair | Trigonal Planar has no lone pair of electrons in the central atom. | A trigonal pyramidal molecule contains a lone pair of electrons in the central atom. |
| Bond angle | The peripheral atoms make an angle of 120° between them. | The angle between atoms of a pyramidal is always less than the angle of a tetrahedron i.e. 109°. It is generally around 107°. |
| Type of repulsion | Trigonal planar displays bond-bond repulsion as every atom exists in a bond only. | Trigonal pyramidal displays both bond-bond as well as bond-lone pair repulsion due to bond and lone pair existence. |
| Repulsion | Repulsion between atoms is less due to the existence of only bond-bond repulsion. | Repulsion between atoms is more due to the existence of both bond-bond and bond-lone pair repulsion. |
Comparison Chart
Comparison Video
The Final Words
Trigonal planar and trigonal pyramidal are geometrical terms used for the three-dimensional positioning of atoms in a molecule. Trigonal planar has one atom at the center and three peripheral atoms at the corners that form an equilateral triangle. On the other hand, trigonal pyramidal has one atom at the apex and three atoms at the base thereby forming a tetrahedron. The atoms of a trigonal planar lie in the same plane while that of trigonal pyramidal lie in different planes. Additionally, trigonal pyramidal has one lone pair of electrons in the central atom while trigonal planar has no suck lone pair. All the bond angles in a planar are 120° whereas these angles in a pyramidal molecule are always less than 109°.
Trigonal Planar vs. Trigonal Pyramidal FAQ
What is the Difference Between Trigonal Planar and Trigonal Pyramidal?
The difference between trigonal planar and trigonal pyramidal can be seen in the structure of a molecule. Trigonal planar does not have a lone pair of electrons while trigonal pyramidal has a lone pair in the central atom. Also, all the atoms of a planar molecule lie in the same plane unlike the atoms of pyramidal.
What makes Trigonal Pyramidal?
The trigonal pyramidal molecular geometry has a central atom at the apex and three atoms attached to it at the base. There are bonded pairs of electrons and a lone pair. This makes the structure a pyramid as the lone pair electron keeps pushing the bonded pairs away, thus pushing them into another plane. An example of a trigonal pyramidal structure is ammonia.
Is PCL3 Trigonal Pyramidal?
Yes, PCl3 or Phosphorus Trichloride is trigonal pyramidal. It has one lone pair of electrons around Phosphorus at the center and three single bonds between phosphorus and chlorine atoms. The bond angle formed is also less than 109° making it a trigonal pyramidal. (Ref. 1)
Why is Phosphine Trigonal Pyramidal?
Phosphine or PH3 is a trigonal pyramidal as phosphorus has three bonded electron pairs and a lone pair attached to it. The lone pair thus shows repulsion to the bonded pairs and pushes them to another plane. Additionally, the bond angle is just 93°.
Which has a Trigonal Pyramidal Structure?
In organic chemistry, the three-dimensional arrangement of atoms in a molecule determines the structure of compounds. There can be bonded pairs and lone pairs. The lone pairs show strong repulsion to the bonded pairs and thus push them the farthest in a separate plane altogether. This is called a trigonal pyramidal structure. Examples are Sulfite ion, chlorate ion, ammonia, xenon trioxide, to name a few.
Why is Ammonia Pyramidal?
Ammonia is a trigonal pyramidal as it has an unshared pair of electrons attached to the central nitrogen atom. The three hydrogen atoms are repelled by the lone pair electrons pushing them as far as around 109° and in a different plane, thus making the shape pyramidal. (Ref. 2)







