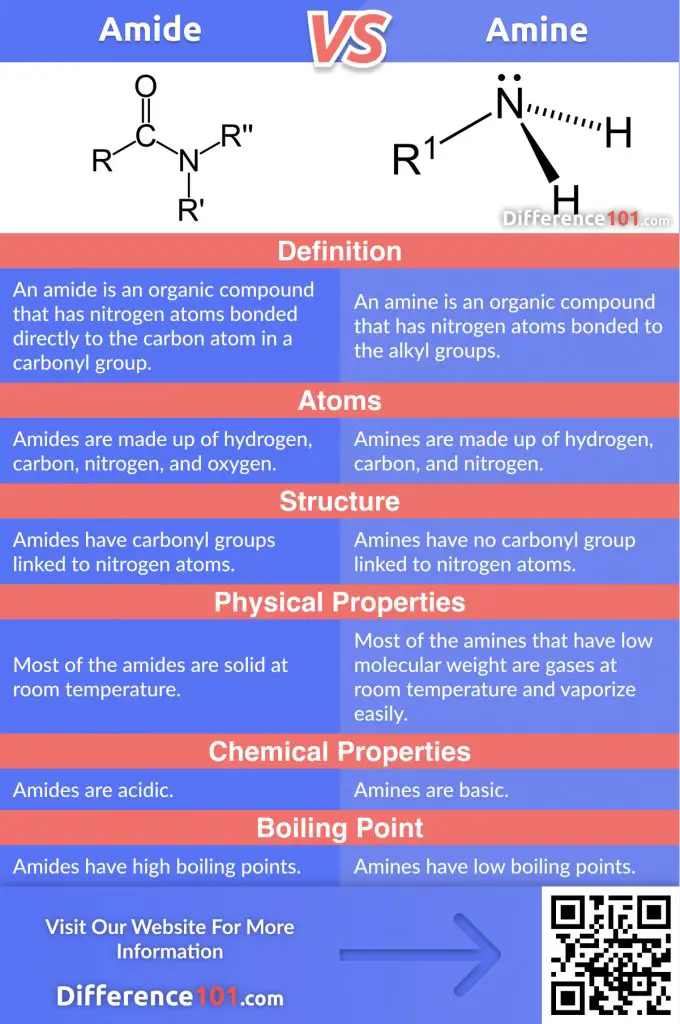
Both amide and amine are formed with the addition of nitrogen in an organic framework. However, the amide vs. amine difference lies in the bond developed by nitrogen present in these compounds. While an amide contains nitrogen atoms bonded to the carbonyl group, an amine contains nitrogen atoms bonded to the alkyl group.
Let’s take a closer look at amide vs. amine:
| Carbonyl groups | Amides have carbonyl groups linked to nitrogen atoms unlike amines |
| Chemical properties | Amides are acidic while amines are basic |
| Boiling point | Amides have a higher boiling point than amines |
| Physical properties | Most amides are solid at room temperature unlike amines |
Table of Contents
What is an Amide?
An amide is an organic compound that has nitrogen atoms bonded directly to the carbon atom in a carbonyl group. These compounds are made up of hydrogen, carbon, nitrogen, and oxygen. They are acidic and most of them are solid at room temperature.
What is an Amine?
An amine is an organic compound that has nitrogen atoms bonded to the alkyl groups. These compounds are made up of hydrogen, carbon, and nitrogen. They are basic and most of them are gases at room temperature.
Amide vs. Amine Pros and Cons
Amide Pros and Cons
Pros of Amides
- They are used in rubber, paper, and textile industries.
- They are used in making body gear.
- They are also used in the manufacturing of pesticides and insecticides.
Cons of Amides
- They are used in the production of toxic plastics.
- Metallic amides are used as explosives that are damaging to the environment.
Amine Pros and Cons
Pros of Amines
- They are used in the pharmaceutical industry.
- They are used in the production of nylon.
Cons of Amines
- They can corrode plastics and metals.
- Some of them are carcinogenic.
Amine vs. Amide Examples
Examples of Amide
Dimethylformamide, acetamide, and benzamide are some examples of amide.
Examples of Amine
Ethanolamines, aniline, and chloramine are some examples of amine.
Amide vs. Amine Similarities Explained
- Both amides and amines contain nitrogen atoms.
- Both are soluble in water due to the -NH group in them.
- Both the compounds can be primary, secondary, and tertiary depending on the number of alkyl groups connected to the nitrogen atom.
- Both amines and amides can be further classified into aromatic and aliphatic compounds.
6 Key Points of Difference Between Amine and Amide Listed Here
There are some notable differences between amines and amides:
| Basis | Amide | Amine |
|---|---|---|
| Definition | An amide is an organic compound that has nitrogen atoms bonded directly to the carbon atom in a carbonyl group. | An amine is an organic compound that has nitrogen atoms bonded to the alkyl groups. |
| Atoms | Amides are made up of hydrogen, carbon, nitrogen, and oxygen. | Amines are made up of hydrogen, carbon, and nitrogen. |
| Structure | Amides have carbonyl groups linked to nitrogen atoms. | Amines have no carbonyl group linked to nitrogen atoms. |
| Physical Properties | Most of the amides are solid at room temperature. | Most of the amines that have low molecular weight are gases at room temperature and vaporize easily. |
| Chemical properties | Amides are acidic. | Amines are basic. |
| Boiling point | Amides have high boiling points. | Amines have low boiling points. |
Comparison Chart
Comparison Video
Amide and Amine FAQ
Can an Amide be an Amine?
An amide can be an amine if heated in a basic or acidic aqueous solution. It will, thus, get hydrolyzed and be converted to amine (Ref. 1). Alternatively, such a conversion can be performed with Hofmann Bromamide Reaction.
What is the Difference Between Amine and Amino?
In organic chemistry, an amine contains an amine functional group whereas amino is the amine functional group itself. In inorganic chemistry, an amine is a functional group that is derived from ammonia (NH3) by replacing its one, two, or three hydrogen atoms with a hydrocarbon. Amines can be primary, secondary, or tertiary but amino can only be primary.
Which is more Basic Amine or Amide?
Amines are more basic. This is because the lone pair electron in an alkylamine is localized on the nitrogen. (Ref. 2)
How can you Identify an Amide?
One can identify an amide by looking at its general structure where a nitrogen atom is connected to a carbonyl carbon atom. An amide shows stronger hydrogen bonds when considering amine vs. amide IR spectra.
What is Amide Used for?
Amides have commercial purposes. Ethanamide and dimethylformamide have strong solvent properties and are, thus, used as sulfa drugs, solvents, and soldering fluxes. Carbamides are synthesized in huge quantities for fulfilling their demand in animal feed, fertilizers, et al.
Are Amines Dangerous?
Not all Amines are dangerous. Some amines are naturally occurring pharmaceuticals and food components. That said, aliphatic amines can be poisonous if exposure to them is for longer periods. Aromatic amines can destroy hemoglobin and are carcinogenic. These can also corrode plastics and metals.
Is NH an Amino group?
Yes, NH is an amino group.
How do you Turn an Amine into an Amide?
The amine can be turned to amide with the process called amidation. In amidation, carboxylic acids are made to react with amines. With the removal of a water molecule during the process, the amide is formed.
Which Amine is More Basic?
A non-conjugated amine is more basic. In other words, the most basic amine is the one that has electron-donating groups. (Ref. 4)
How is an Amide Formed?
Amide is formed by adding ammonia to a carboxylic acid.
The Final Words
The addition of nitrogen in an organic framework leads to the formation of either an amide or an amine. The bond developed by the nitrogen atom present in these compounds determines the difference between them. The nitrogen atom present in an amide compound bonds directly to the carbon atom in a carbonyl group. On the other hand, the nitrogen atom present in an amine compound bonds to the alkyl groups. Additionally, amides are acidic while amines are basic.
References
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/21%3A_Carboxylic_Acid_Derivatives-_Nucleophilic_Acyl_Substitution_Reactions/21.07%3A_Chemistry_of_Amides
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Vollhardt_and_Schore)/21%3A_Amines_and_Their_Derivatives/21.04%3A_Acidity__and__Basicity__of_Amines
- https://www.britannica.com/science/amine
- https://www.masterorganicchemistry.com/2017/04/26/5-factors-that-affect-basicity-of-amines/#three







